import tellurium as te
import matplotlib.pyplot as plt
from matplotlib.animation import FuncAnimation, FFMpegFileWriter
r = te.loada('''
J1: $Xo -> S1; e1*(k10*Xo - k11*S1);
J2: S1 -> S2; e2*(k20*S1 - k21*S2);
J3: S2 -> S3; e3*(k30*S2 - k31*S3);
J4: S3 -> S4; e4*(k40*S3 - k41*S4);
J5: S4 -> S5; e5*(k50*S4 - k51*S5);
J6: S5 -> S6; e6*(k60*S5 - k61*S6);
J7: S6 -> S7; e7*(k70*S6 - k71*S7);
J8: S7 -> S8; e8*(k80*S7 - k81*S8);
J9: S8 -> S9; e9*(k90*S8 - k91*S9);
J10: S9 -> S10; e10*(k100*S9 - k101*S10);
J11: S10 -> S11; e11*(k110*S10 - k111*S11);
J12: S11 -> S12; e12*(k120*S11 - k121*S12);
J13: S12 -> S13; e13*(k130*S12 - k131*S13);
J14: S13 -> S14; e14*(k140*S13 - k141*S14);
J15: S14 -> S15; e15*(k150*S14 - k151*S15);
J16: S15 -> S16; e16*(k160*S15 - k161*S16);
J17: S16 -> S17; e17*(k170*S16 - k171*S17);
J18: S17 -> S18; e18*(k180*S17 - k181*S18);
J19: S18 -> S19; e19*(k190*S18 - k191*S19);
J20: S19 -> $X1; e20*(k200*S19 - k201*X1);
k10 = 0.86; k11 = 0.24
e1= 1; k20 = 2.24; k21 = 0.51
e2= 1; k30 = 2.00; k31 = 0.49
e3= 1; k40 = 2.54; k41 = 0.83
e4= 1; k50 = 3.05; k51 = 0.97
e5= 1; k60 = 1.29; k61 = 0.10
e6= 1; k70 = 1.94; k71 = 0.20
e7= 1; k80 = 3.01; k81 = 0.17
e8= 1; k90 = 0.64; k91 = 0.19
e9= 1; k100 = 1.96; k101 = 0.42
e10= 1; k110 = 2.47; k111 = 0.63
e11= 1; k120 = 4.95; k121 = 0.51
e12= 1; k130 = 4.78; k131 = 0.70
e13= 1; k140 = 3.77; k141 = 0.78
e14= 1; k150 = 1.93; k151 = 0.37
e15= 1; k160 = 3.87; k161 = 0.96
e16= 1; k170 = 0.83; k171 = 0.37
e17= 1; k180 = 1.82; k181 = 0.69
e18= 1; k190 = 2.62; k191 = 0.83
e19= 1; k200 = 4.85; k201 = 0.67
e20= 1; Xo = 10.00
X1 = 0
S1 = 0; S2 = 0; S3 = 0; S4 = 0;
S5 = 0; S6 = 0; S7 = 0; S8 = 0;
S9 = 0; S10 = 0; S11 = 0; S12 = 0;
S13 = 0; S14 = 0; S15 = 0; S16 = 0;
S17 = 0; S18 = 0; S19 = 0;
''')
# I'm running this on windows so I had to install the ffmpeg binary
# in order to save the video as an mp4 file
# for more info go tot his page:
# https://suryadayn.medium.com/error-requested-moviewriter-ffmpeg-not-available-easy-fix-9d1890a487d3
plt.rcParams['animation.ffmpeg_path'] ="C:\\ffmpeg\\bin\\ffmpeg.exe"
endTime = 18
fig, ax = plt.subplots(figsize=(8, 5))
ax.set(xlim=(-1, r.getNumIndFloatingSpecies()), ylim=(0, 18))
m = r.simulate(0, endTime, 100)
label = ax.text(15.6, 19, 'Time=', ha='center', va='center', fontsize=18, color="Black")
bars = ax.bar(r.getFloatingSpeciesIds(), m[0,1:], color='b', alpha = 0.5)
def barAnimate(i):
label.set_text('Time = ' + f'{m[i,0]:.2f}')
for bar, h in zip(bars, m[i,1:]):
bar.set_color ('r')
bar.set_height(h)
anim = FuncAnimation(fig, barAnimate, interval=50, frames=100, repeat=False)
# You can save themp4 file anywhere you want
anim.save('c:\\tmp\\animation.mp4', fps=30)
plt.draw()
plt.show()
This is the resulting video:
Sunday, December 17, 2023
Animating pathway
Wednesday, December 13, 2023
Update to teUtils
I just updated teUtils to version 2.9
UPDATE: The documentation was broken, now fixed. This is a set of utilities that can be used with our simulation environment Tellurium
The update includes some updates to the build synthetic networks functionality.
The new version adds 'ei*(' terms to mass actions rate laws, eg
S1 -> S2; e1*(k1*S1 - k2*S2)
This makes it easier to compute control coefficients with repect to 'e'
I also added a new mass-action rate of the form:
v = k1*A(1 - (B/A)/Keq1)
These can be generated using the call:
model = teUtils.buildNetworks.getLinearChain(10, rateLawType="ModifiedMassAction")
Useful if you want to more easily control the equilbrium constant for a reaction.
Here is an example of a three step random linear chain:
model = teUtils.buildNetworks.getLinearChain(10, rateLawType="ModifiedMassAction")
print (model)
J1: $Xo -> S1; k1*Xo*(1 - (S1/Xo)/Keq1);
J2: S1 -> S2; k2*S1*(1 - (S2/S1)/Keq2);
J3: S2 -> $X1; k3*S2*(1 - (X1/S2)/Keq3);
k1 = 1.42; Keq1 = 3.61
e1 = 1; k2 = 1.42; Keq2 = 7.91
e2 = 1; k3 = 3.50; Keq3 = 5.64
e3 = 1; Xo = 5.00
X1 = 0
S1 = 1E-6; S2 = 1E-6;
Monday, December 4, 2023
Executing tellurium models in a sphinx document
I needed to be able to generate simulation plots for a sphinx document but I didn't want to have to generate the plots separately and then include them manually. I wanted the simulations done from within sphinx so that they would be automatically included when building the document. The key to this is to add the following to the list of extensions in the sphinx conf.py file:
extensions.append ('matplotlib.sphinxext.plot_directive')
To use it in a sphinx document use the plot directive, for example:
.. plot::
:include-source:
import tellurium as te
r = te.loada ('''A -> B; k1*A; k1=0.1; A = 10''')
m = r.simulate (0, 40, 100)
r.plot()
It assumes you have matplotlib installed in your python setup (I am using Windows 10, Python 3.11). Further information can be found here:
https://matplotlib.org/stable/api/sphinxext_plot_directive_api.html
How to execute python code in a sphinx document
Wednesday, November 29, 2023
A simple linear pathway
import tellurium as te
import matplotlib.pyplot as plt
r1 = te.loada("""
-> P; k0 + k1*S
P ->; k2*X*P
-> X; k3*S
X ->; k4*X
k1 = 1; k2 = 1
k3 = 1; k4 = 1
# Set basal rate to zero
k0 = 0; S = 1
# Change signal, P won't change
at time > 10: S = S*2
# Change basal rate and set S back to what it was
at time > 25: k0 = 0.3, S = 1;
# Change signal, this time P will change
at time > 40: S = S*2
""")
m = r1.simulate(0, 60, 200, ['time', 'P', 'S'])
plt.plot (m['time'], m['P'], label='P')
plt.plot (m['time'], m['S'], label='S')
plt.text(2, 0.75, "Basal = 0")
plt.text(9, 0.9, "Change S")
plt.text(14, 1.2, "P restored")
plt.text(20, 0.75, "Set basal > 0")
plt.text(35, 0.9, "Change S, P not restored")
plt.legend()
Sunday, November 12, 2023
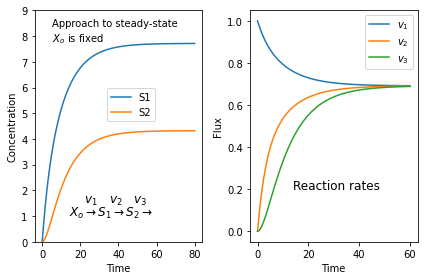
Plotting a steady-state plot
It's always surprising to find students who don't quite get what a steady-state is. Here is a simulation of a three step pathway that might help. You'll need to install tellurium for this to work.
$X_o \stackrel{v_1}{\rightarrow} S_1 \stackrel{v_2}{\rightarrow} S_2 \stackrel{v_3}{\rightarrow}$
we assume that $X_o$ is fixed and does not change in time. I don't care where $S_3$ goes.
If we start with zero concentrations for $S_1$ and $S_2$ we get the following plots. The left plot shows the change in concentration and the right plot the reaction rates. Note that all three reaction rates approach the same rate since at steady-state all rates must be equal.
import tellurium as te
import matplotlib.pyplot as plt
r = te.loada("""
J1: $Xo -> S1; k1*Xo - k2*S1
J2: S1 -> S2; k3*S1 - k4*S2
J3: S2 -> ; k5*S2
k1 = 0.1; k2 = 0.04
k3 = 0.14; k4 = 0.09
k5 = 0.16
Xo = 10
""")
m = r.simulate (0, 60, 100)
plt.subplot (1,2, 1)
plt.plot(m['time'], m['[S1]'], label='S1')
plt.plot(m['time'], m['[S2]'], label='S2')
plt.ylim((0, 9))
plt.xlabel('Time')
plt.ylabel('Concentration')
plt.text(5, 8.4, 'Approach to steady-state')
plt.text(5, 7.8, '$X_o$ is fixed')
plt.text(14, 1.5, r'$\quad\ v_1 \quad v_2 \quad v_3$', fontsize = 12)
plt.text(14, 1, r'$X_o \rightarrow S_1 \rightarrow S_2 \rightarrow$', fontsize = 12)
plt.legend(bbox_to_anchor=(0.4, 0.5))
# Next generate the reaction rates
r.reset()
m = r.simulate (0, 40, 100, ['time', 'J1', 'J2', 'J3'])
plt.subplot (1,2, 2)
plt.plot(m['time'], m['J1'], label='$v_1$')
plt.plot(m['time'], m['J2'], label='$v_2$')
plt.plot(m['time'], m['J3'], label='$v_3$')
plt.xlabel('Time')
plt.ylabel('Flux')
plt.text(14, 0.2, r'Reaction rates', fontsize = 12)
plt.legend()
plt.tight_layout()
plt.show()
Tuesday, September 19, 2023
1888 Math Exam
Nothing to do with cells or modeling, but I recently purchased a 1885 copy of John Casey's rendition of Euclid's Elements. John Casey was born in Limerick, Ireland, and became a lecturer in mathematics at University College Dublin. He wrote one of the more well-known editions of Euclid's elements. In his preface he states:
"This edition of the Elements of Euclid, undertaken at the request of the principals of some of the leading Colleges and Schools of Ireland, is intended to supply a want much felt by teachers at the present day—the production of a work which, while giving the unrivalled [sic] original in all its integrity, would also contain the modern conceptions and developments of the portion of Geometry over which the Elements extend."
You can find a copy at Project Gutenberg (https://www.gutenberg.org/ebooks/21076) if you are curious.
What's interesting about the book I received, is not so much the geometry, but what I found inside.
Inside was a copy of a math Pass Examination paper from 1888, presumably from University College Dublin but at least somewhere in Ireland (I purchased the book from Dublin). Update: There are two inscriptions in the book. The first is a simple "W.H Dunlop May 1886". The second is more interesting and written in a very elegant cursive style where the book was transferred two years later to: Michael J Buckley. Catholic University Dublin. 19.1.'88. This is the name of the first university dedicated to accepting Catholics from Ireland (Trinity College was the Anglican University founded by Elizabeth I, so you can imagine the problem). However, the university didn't do too well financially and had problems awarding degrees because it didn't have a royal charter. In 1908/1909, it became the University College Dublin with its own charter.
The exam had 10 questions, one involving reaping a field, another about selling a horse, and another about carpeting a room. The remaining 7 are pure math questions, with a number of them being numerical estimation questions, eg, roots, squares etc. I think modern students could do these questions, though they might struggle with the numeric computations unless they have a calculator at hand. I took a photograph of the exam for all to see:
Wednesday, May 3, 2023
Optimal distribution of enzymes that maximizes flux
import tellurium as te
import roadrunner
import matplotlib.pyplot as plt
r = te.loada("""
J1: $Xo -> S; e1*(k1*Xo - k2*S)
J2: S ->; e2*k3*S
Xo = 1
k1 = 0.5; k2 = 0.2
k3 = 0.45
e1 = 0.01; e2 = 0.99
""")
r.steadyState()
x = []; y = [];
for i in range (49):
r.e1 = r.e1 + 0.02
r.e2 = 1 - r.e1 # Total assumed to be one
r.simulate()
r.steadyState()
x.append (r.e1)
y.append (r.J1)
plt.figure(figsize=(12,10))
plt.grid(b=True, which='major', color='#666666', linestyle='-')
plt.grid(b=True, which='minor', color='#999999', linestyle='-', alpha=0.2)
plt.minorticks_on()
plt.plot (x, y, linewidth = 2)
plt.xlabel('$e_1$', fontsize=36)
plt.ylabel('Flux', fontsize=36)
The following graph show the results from the simulation (the font sizes might be on the big size if you have a low res monitor):
You can see the flux reaching a maximum at around $e_1 = 0.6$, meaning also that $e_2 = 0.4$ in order to keep the total fixed. The actual position of the peak wil depend on the rate constants in the rate expressions.
Let's assume we are at the maxium. The slope, $dJ/de_1$, at the maxium is obviously zero. That means if we were to move a small amount of protein from $e_1$ to $e_2$ the flux won't change. We can write this experiment in terms of the two flux control coefficients:
$$ \frac{\delta J}{J} = 0 = C^J_{e_1} \frac{\delta e_1}{e_1} + C^J_{e_2} \frac{\delta e_2}{e_2} $$
However, we know that in this particlar experiment the change in $e_1$ is the same but oppisite to the change in $e_2$. That is $\delta e_1 + \delta e_2 = 0$ or
$$ \delta e_1 = -\delta e_2$$
Replacing $e_2$ with $-\delta e_1$ gives:
$$ \frac{\delta J}{J} = 0 = C^J_{e_1} \frac{\delta e_1}{e_1} - C^J_{e_2} \frac{\delta e_1}{e_2} $$
$$ \frac{\delta J}{J} = 0 = C^J_{e_1} \frac{1}{e_1} - C^J_{e_2} \frac{1}{e_2} $$
$$ C^J_{e_1} \frac{1}{e_1} = C^J_{e_2} \frac{1}{e_2} $$
Giving the final result:
$$ \frac{C^J_{e_1}}{C^J_{e_2}} = \frac{e_1}{e_2} $$
That is, when the protein distribution is optimized to maximize the flux, the flux control coefficients are in the same ratio as the ratio of enzyme amounts. This generalizes to any size pathway with multiple enzymes.
This gives us a tantgilizing suggestion that we can obtain the flux control coeficients just by measuring the protein levels.
There is obviously a lot more one can write there and maybe I do that in future blogs but for now you can get further information:
S. Waley, “A note on the kinetics of multi-enzyme systems,” Biochemical Journal, vol. 91, no. 3, p. 514, 1964.
J Burns: “Studies on complex enzyme system.” https://era.ed.ac.uk/handle/1842/13276, 1971 (page 141-) I have a LaTeX version at: https://github.com/hsauro/JumBurnsThesis
Guy Brown, Total cell protein concentration as an evolutionary constraint on the metabolic control distribution in cells,” Journal of theoretical biology, vol. 153, no. 2, pp. 195–203, 1991.
E. Klipp and R. Heinrich, “Competition for enzymes in metabolic pathways:: Implications for optimal distributions of enzyme concentrations and for the distribution of flux control,” Biosystems, vol. 54, no. 1-2, pp. 1–14, 1999
Sauro HM Systems Biology: An Introduction to Metabolic Control Analysis, 2018
Friday, April 21, 2023
Relationship of fluxes to enzyme levels in a metabolic pathway
Someone asked me the other day what the relationship was between the steady-state fluxe through a reaction and the coresponding level of enzyme. Someone else suggested that there would be a linear, or proportional relatinship between a flux and the enzyme level. However, this can’t be true, at least at steady. Considder a 10 step linear pathway. At steady-state each step in the pathway will, by defintion, carry the same flux. This is true even if each step has a different enzyme level. Hence the relationship is so simple. In fact the flux a given step carries is a systemic properties, dependent on all steps in the pathway. As an experiment I decided to do a simulation on some synthetic netowrks with random parameters and enzyme levels. For this exmaple I just used a simple rate law of the form: $$ v = e_i (k_1 A - k_2 B) $$ For a bibi reaction, A + B -> C + D, the coresponding rate law would be: $$ v = e_i (k_1 A B - k_2 C D) $$ a similar picture would be seen for the unibi and biuni reactions. Using our teUtils package I generated random networks with 60 species and 150 reactions. The reactions allowed are uiui-uinbi, biui or bibi. I then randomized the values for the enzymne levels $e_i$ and computed the steady-state flux. I used the following code to do the analysis. I have a small loop that generates 5 random models but obviously this number can be changed. I generate a random model, load the model into roadrunner, randomize the values for the $e_i$ parameters between 0 and 10, compute the steady-state (I do a presimulation to help things along) and collect the corresponding $e_i$ and flux values. Finally I plot each pair in a scatter plot.
import tellurium as te
import roadrunner
import teUtils as tu
import matplotlib.pyplot as plt
import random
for i in range (5):
try:
J = []; E = []
antStr = tu.buildNetworks.getRandomNetwork(60, 150, isReversible=True)
r = te.loada(antStr)
n = r.getNumReactions()
for i in range (n):
r.setValue ('E' + str (i), random.random()*10)
m = r.simulate(0, 200, 300)
r.steadyState()
for i in range (n):
J.append (abs (r.getValue ('J' + str (i))))
E.append (r.getValue ('E' + str (i)))
plt.figure(figsize=(12, 8))
plt.plot(E, J, '.')
except:
print ('Error: bad model')
The results for five random networks is shown below. Note the x axis is the enzyme level and the y axis the corresponding steady-state flux through that enzyme. It's intersting to see that there is a rough correlation between enzyme amount and the corresponding flux, but its not very strong. Many of the points are just scattered randomly with some showing a definite correlation. The short answer is the realtinship is not so simple.
Wednesday, March 1, 2023
How not to Comment Code
"Ten simple rules for tackling your first mathematical models: A guide for graduate students by graduate students" by Korryn Bodner et al
https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1008539
One thing that struck me was Rule 5 on coding best practices with commenting being one of the discussion points. What struck me was their screen shot of a documented function shown below (in R):
My take on commenting is that it should be used to add human readable metadata on elements of a program that are not immediately obvious.
Most of the time, code should be sufficiently readable to indicate what it's doing. Obviously some languages are better than others when desribing an algorithm but it is also dependent on the programmer. I've seen code written in clear languages that are unintelliglbe, but I've also seen code written in poorly expressible languages that are easily readable. Although the programming language itself can influence code reability I think the programmer has much more influence.
But back to Rule 5. In the example you'll see something like:
# calculate the mean of the data
u <- mean (x)
This is completely redundant, as the coding states what it is going to do. In fact the authors comment every line like this. If anythng, I think the extent of comments actually hinders the reabilty of the code. The code itself is mostly clear as to what it is doing. There may be a justification to include a comment on next line that computes the standard error because the variables names are so badly chosen, e.g what does the following line do:
s <- sd(x)
sd might stand for standard deviation but the rest of the line offers no clue. If it had been written as:
standardDeviation <- sd(x)
It would have been much clearer, instead the authors add a comment to make up for poor choice of variable names. They also give the function itself a nondescriptive name, in this case ci. It would have been better to write the function using getConfidenceInterval or similar:
getConfidenceInterval <- f (data) {
etc
Tuesday, January 24, 2023
Euclid's Elements
It occurred to me that something similar could be done with deriving the properties of biochemical networks. For example, we might define the following three primitives:
I. Species
II. Reaction
III. Steady-state
We might then define the following axioms:
I. A species has associated with it a value called the concentration, x_i.
II. All concentrations are positive.
III. A reaction has a value associated with it called the reaction rate, v_i.
IV. Reaction rates can be negative, zero, or positive.
V. A reaction transforms one or more species (reactants) into one or more other species (products).
VI. The reaction rate is a continuous function of the reactants and products.
VII. The rate of change of a species can be described using a differential equation, dx/dt
VIII. All steps are reversible unless otherwise stated (may this can be derived?)
etc
Given these axioms, we could build a series of propositions. This might be an interesting exercise to do. Some of the more obvious propositions would be the results from metabolic control analysis, such as the summation and connectivity theorems.