May 30, 2011 2:27 pm
In part 1 of this series, a brief history of negative feedback was given. Here we will look at the advantages and disadvantages of negative feedback, particularly in relation to signal transmission.
Amplifiers
Amplifiers used by electrical engineers are designed to magnify the current or voltage in an electrical circuit. A simple example is the voltage amplifier (i.e. voltage-controlled voltage source) which samples the voltage in one part of a circuit and produces a proportionally larger voltage in another. Of critical importance to an amplifier is not so much the amplification factor itself but how accurate the amplification is. There is no such thing however as the perfect amplifier and real amplifiers, electrical or biological will introduce errors or distortions into the signal. These distortions can be classed into three types:
- Frequency distortion
- Phase distortion
- Harmonic distortion
Frequency distortion is do to the fact that amplifiers do not amplify signals at different frequencies to the same extend. The term bandwidth is used to indicate the range of frequencies over which a given amplifier can faithfully amplify a signal.
The second source of distortion, that is phase distortion, is do to the fact that as the amplifier operates it will add delays into the signal. The amount of delay will often be a function of the signal frequency.
Finally, harmonic distortion is due to the fact that amplifiers do not amplify a signal by a fixed amount. That is the amplifier will have some nonlinear behavior which will often be a function of signal frequency.
In the 1920s, such distortions were a huge problem to the new telecommunications industry and it was Harold Black's solution to use negative feedback that solved the problem.
Negative Feedback
In order to understand how negative feedback can improve the performance of a signal amplifier, we must consider a very simple example. The figure below comes from the paper: "MAPK Cascades as Feedback Amplifiers"
Let us consider only the steady-state behavior of the system. The input is given by $u$, the output by $y$, the error $e$, and the disturbance by $d$. The input is the signal we want to magnify and the magnified version of the input is the output, $y$. Some of the output we feed back via $F$ to the input, where we subtract it from the input, $u$. $A$ is the amplifier itself. We will ignore the disturbance $d$ for the moment. The way to look at this diagram mathematically is that any arrow coming out of a block is the product of the block and the arrow coming into the block. For example, consider the output, $y$. This output is a result of the amplifier, $A$, magnifying the error, $e$, that is $y = A e$. What about $e$? $e$ is the result of subtracting $F y$ from $u$. From these statements we can write the following two equations:
$$ y = A e $$ $$ e = u - F y $$ From these two equations we can eliminate $e$ to find: $$ y = \frac{A u}{1 + A F} $$Calling $G = A/(1 + A F)$ the system gain, we have simply, $y = G u$. Comparing $G$ with $A$, it should be clear that the feedback reduces the gain of the amplifier. Further, if the loop gain $A F$ is large ($A F \ge 1$), then
$$ G \approx \frac{A}{A F} = \frac{1}{F} $$That is, as the gain $A F$ increases, the system behavior becomes more dependent on the feedback loop and less dependent on the amplifier itself. But so what? Three things are apparent from this simple analysis, the first is that any variation is $A$ has no effect on the operation of the system, that is because $G$ is independent of $A$.From a practical point of view, the manufacturing tolerance of $A$ doesn't have to be so high which makes it possible to make cheap $A$s. Instead the designer need only provide a stable feedback mechanism, in electronics this is in the form of cheap but high tolerance resistors.
Secondly advantage of having feedback, if we introduce a disturbance, $d$, into the output we find that in the presence of feedback, the influence of the disturbance decreases. Finally, and this is the real magic, any nonlinearity present in the amplifier $A$, is eliminated (or at least greatly reduced), this means that our feedback amplifier is very good at faithfully magnifying the input signal, exactly what we want from an amplifier (Proofs of these assertions can be found in the original papers, eg see [3]). In summary a feedback amplifier provides the following desirable characteristics: 1. Increased robustness with respect to internal perturbations. 2. Insulation from external perturbation, resulting in functional modularization. 3. A linear graded response over an extended operating range.A word about the source of the internal variations in $A$. There are two primary sources, the first is manufacturing variability, that is not every amplifier is exactly the same as it comes off the assembly line. Secondly, when the circuit operates, it heats up and this introduces thermal noise which feeds noise directly into the circuit. Also repeated heating and cooling can cause the amplifier components to slow change in behavior. All these sources of variation can be greatly reduced by adding a negative feedback loop.
What has this got to do with MAPK? The MAPK cascade has many features that are similar to a negative feedback amplifier. The input is from a small signal at the receptor, The amplifier part, that is the three phosphorylation cycles act as amplifiers with high gain. A negative feedback wraps the entire structure and finally, in the last stage, the protein ERK2, must diffuse to the nucleus to make any difference. This represents a disturbance in the output signal. The noise in the amplifier part of MAPK can comes from a number of sources. First, there may be natural allelic variation in the cascade proteins but perhaps more significant is the stochastic noise that occurs in transcription and translation which means that the mean concentration of the cascade proteins vary over time. Negative feedback will greatly reduce the effect the variations have on the performance of MAPK.
There is more to a negative feedback amplifier than described here but the most important points are described.
References
1. Quantitative analysis of signaling networks. Sauro HM, Kholodenko BN, Prog Biophys Mol Biol. 2004 Sep;86(1):5–43.
2. The Computational Versatility of Proteomic Signaling Networks, Sauro HM In: Current Proteomics, Vol. 1, Bentham Science Publishers Ltd. (2004) , p. 67-81.
3. MAPK Cascades as Feedback Amplifiers Sauro HM, Ingalls B



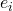 , often through changes in enzyme concentration. This definition assumes that the enzyme concentration is under the direct control of the experimenter and as such can be classed as a parameter. This assumes that a change on the level of the enzyme does not change the level of enzymes. This assumption will not always necessarily be true, in which case the control coefficient can be generalized to be independent of any particular parameter. For now however, with loss of generality, that we can change the enzyme concentration without affecting other enzymes. We define the flux control coefficients as follows:
, often through changes in enzyme concentration. This definition assumes that the enzyme concentration is under the direct control of the experimenter and as such can be classed as a parameter. This assumes that a change on the level of the enzyme does not change the level of enzymes. This assumption will not always necessarily be true, in which case the control coefficient can be generalized to be independent of any particular parameter. For now however, with loss of generality, that we can change the enzyme concentration without affecting other enzymes. We define the flux control coefficients as follows:![Rendered by QuickLaTeX.com \[C^J_{e_i} = \frac{dJ}{de_i} \frac{e_i}{J} \right) = \frac{d\ln J}{d\ln e_i}\]](https://web.archive.org/web/20110713122902im_/http://blog.analogmachine.org/wp-content/ql-cache/quicklatex.com-ee9218b57860adc4739cea3643b416e7_l3.png)
 of step
of step  is given by:
is given by:![Rendered by QuickLaTeX.com \[C^J_{v_i} = \left( \frac{dJ}{dp} \frac{p}{J} \right) \bigg/ \left( \frac{dv_i}{dp}\frac{p}{v_i} \right) = \frac{d\ln J}{d\ln v_i}\]](https://web.archive.org/web/20110713122902im_/http://blog.analogmachine.org/wp-content/ql-cache/quicklatex.com-35aa60f381cf80334428c8df7746ad9e_l3.png)
 , used to perturb the reaction step. A very important property of all control coefficients is that they can only be measured in the intact system. It is not possible to isolate an enzyme and try to measure its control coefficient. The effect that a particular parameter, for example
, used to perturb the reaction step. A very important property of all control coefficients is that they can only be measured in the intact system. It is not possible to isolate an enzyme and try to measure its control coefficient. The effect that a particular parameter, for example ![Rendered by QuickLaTeX.com \[C^S_{e_i} = \frac{dS}{de_i} \frac{e_i}{S} \right) = \frac{d\ln S}{d\ln e_i}\]](https://web.archive.org/web/20110713122902im_/http://blog.analogmachine.org/wp-content/ql-cache/quicklatex.com-33c41be106711b94f49345310d622ba7_l3.png)
![Rendered by QuickLaTeX.com \[C^S_{v_i} = \frac{dS}{dv_i} \frac{v_i}{S} \right) = \frac{d\ln S}{d\ln v_i}\]](https://web.archive.org/web/20110713122902im_/http://blog.analogmachine.org/wp-content/ql-cache/quicklatex.com-a5b83803b73f5028ada49f360eca8a8c_l3.png)
![Rendered by QuickLaTeX.com \[C^J_{e_i} = \left. \frac{\delta J}{J} \right/ \frac{\delta e_i}{e_i} \approx \frac{J\%}{ e_i\% }\]](https://web.archive.org/web/20110713122902im_/http://blog.analogmachine.org/wp-content/ql-cache/quicklatex.com-c2599bb01d6a9c22a5f007b869a60f1a_l3.png)

![Rendered by QuickLaTeX.com \[\left\{\left\{y[t]\to \frac{e^{(k_1-k_2) t} k_1+k_2-2 e^{(k_1-k_2) t} k_2}{k_1-k_2}\right\}\right\}\]](https://web.archive.org/web/20140419132730im_/http://blog.analogmachine.org/wp-content/ql-cache/quicklatex.com-5a3d0d91afb9dd5f52f8e3fb6a19b4ed_l3.png)
![Rendered by QuickLaTeX.com \[\left\{\left\{A[t]\to \frac{v_o-e^{-k_1 t} v_o}{k_1},B[t]\to \frac{\left(k_1-e^{-k_3 t} k_1+\left(-1+e^{-k_1 t}\right) k_3\right) v_o}{(k_1-k_3) k_3}\right\}\right\}\]](https://web.archive.org/web/20140419132730im_/http://blog.analogmachine.org/wp-content/ql-cache/quicklatex.com-9acc3b8f5d7694c87431ca0a73a834cf_l3.png)